Additional information
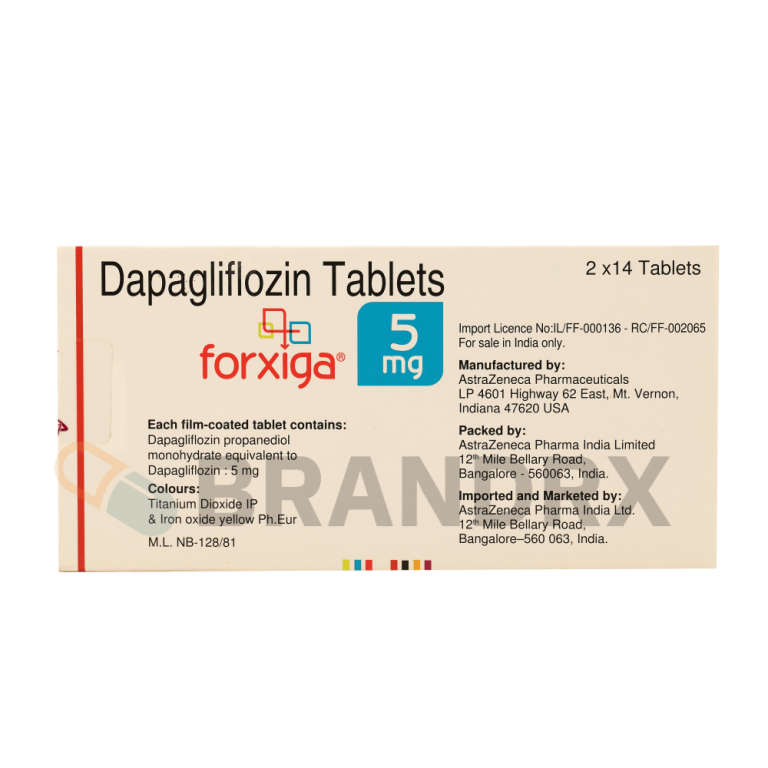
| Active substance | Dapagliflozin |
|---|---|
| Water Retention | Can cause some diuresis, generally does not cause significant water retention |
| Hepatotoxicity | No significant hepatic toxicity reported |
| Lab Test | Monitoring blood glucose levels and renal function tests are recommended |
| Strength | 5mg |
| Also known as | BMS-512148 |
| Blood pressure | Can modestly reduce blood pressure |
| Trade name | Farxiga, Xigduo XR (combination with metformin) |
| Storage conditions | Store at room temperature away from moisture and heat |
| Chemical name | (2S,3R,4R,5S,6R)-2-(4-chloro-3-(4-ethoxybenzyl)phenyl)-6-(hydroxymethyl)tetrahydro-2H-pyran-3,4,5-triol |
| Formula | C21H25ClO6 |
| Substance class | SGLT2 inhibitor |
| Main action | Reduces blood glucose by inhibiting sodium-glucose transport protein 2 in the kidneys |
| Half-life | Approximately 12.9 hours |
| Dosage (medical) | Typically 10 mg once daily |
| Dosage (sports) | Not applicable |
| Effects | Lowers blood sugar, aids in weight loss |
| Side effects | Risk of genital infections, urinary tract infections, possible dehydration, rare cases of diabetic ketoacidosis |
| Use in sports | Not typically used in sports |
| Manufacturer | AstraZeneca Pharma Ltd. |
| Packing | 14 tabs/blister |















Reviews
There are no reviews yet.